Technology


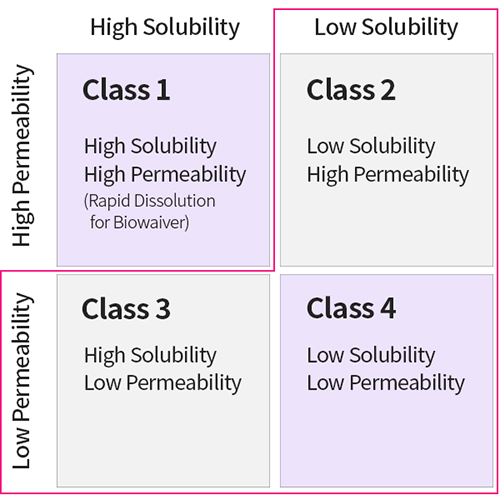
Biopharmaceutics Classification System
BASF Pharma Solutions Solubilization Symposium in Ludwigshafen, Germany (October 2017)
An estimated 90% of APIs in global R&D pipelines possess promising therapeutic potential, yet poor solubility: approximately 70% for Class 2 and 20% for Class 4.
Poor bioavailability is another issue to be solved. It leads to suboptimal drug delivery, poor efficacy, side-effects, cost burden, increased risks of toxicity, revenue miss, R&D expense loss, and more.
An estimated 40% of drugs in the market have poor solubility.
The number of low-solubility drugs has increased since mid 90’s and this trend is continuing.

Disruptive Bioavailability Innovation with Dramatically-Enhanced Permeability
& Higher Drug Loading well beyond its Solubility
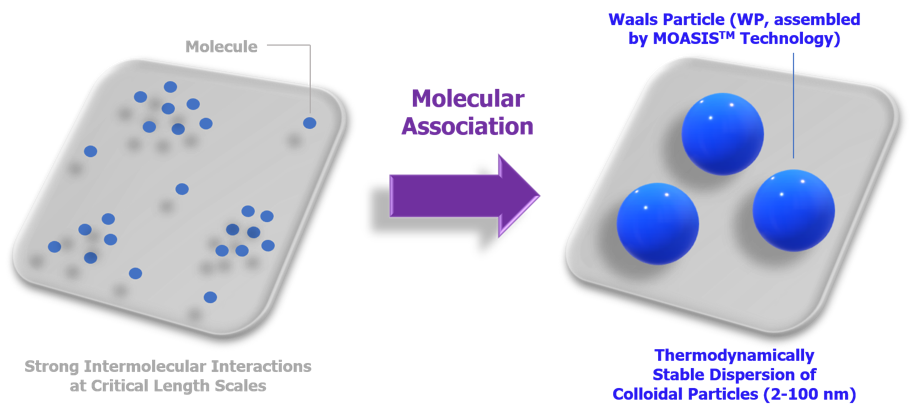
The platform technology MOASIS™ enables the construction of novel molecular clusters
from small molecules within critical length scales.

MOASIS™ is a robust & versatile platform technology.
![]() Bottom-up approach to a molecular cluster formation.
Bottom-up approach to a molecular cluster formation.
![]() Physical method independent of chemical and structural property of the drug.
Physical method independent of chemical and structural property of the drug.
![]() Adjustable particle size and surface property, available in dispersion or in solid form.
Adjustable particle size and surface property, available in dispersion or in solid form.
![]() Easy to scale-up and suitable for mass production.
Easy to scale-up and suitable for mass production.
![]() Improved bioavailability through the enhancement of solubility and permeability.
Improved bioavailability through the enhancement of solubility and permeability.

